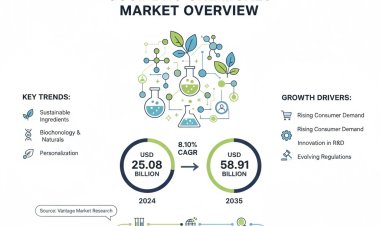
Global Allergy Immunotherapy Market Size to Reach $3.33 Billion at a CAGR of 9.2% by 2030
Vantage Market Research expects the Allergy Immunotherapy Market to reach USD 3.33 Billion by 2030, exhibiting a growth rate (CAGR) of 9.2% during 2024-2030.

The Global Allergy Immunotherapy Market size reached USD 1.63 Billion in 2022. Vantage Market Research expects the market to reach USD 3.33 Billion by 2030, exhibiting a growth rate (CAGR) of 9.2% during 2024-2030.
Table of Contents
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Introduction
Allergies occur when the immune system reacts abnormally to usually harmless substances, affecting a percentage of the population ranging from 10 to 40%. Asthma and allergic rhinitis are examples of respiratory allergies. It is noteworthy that between 10 and 40 percent of patients with allergic rhinitis also have allergic asthma, and between 60 and 80 percent of persons with asthma also have persistent symptoms of allergic rhinitis. Allergy rhinitis is a known risk factor for poor asthma management because individuals with severe allergic rhinitis and asthma have poor asthma control rates that are 4–5 times greater than those without the condition. Given the significant impact of allergic rhinitis and its frequently coexisting nature with asthma, finding treatment options that address the underlying cause of allergies while also benefiting both conditions is crucial. The only treatment that addresses the underlying cause of allergies is Allergy Immunotherapy or AIT. Thus, it is anticipated that, the Global Allergy Immunotherapy Market is poised to reach a valuation of USD 3.33 Billion by 2030, experiencing exponential growth of 9.20% over the next seven years. This therapy gradually increases the immune system's tolerance to the allergenic substance through repeated administrations, resulting in a modulation of the immunological response.
Request Sample Report of Allergy Immunotherapy Market @ https://www.vantagemarketresearch.com/allergy-immunotherapy-market-2360/request-sample
Top Companies in Global Allergy Immunotherapy Market
- Merck KGaA (Germany)
- Anergis SA (Switzerland)
- ASIT Biotech (Belgium)
- Jubilant Pharma (Hollister Allergy) (U.S.)
- Leti Pharma (Spain)
- Stallergenes Greer (Switzerland)
- ALK-Abello A/S (Denmark)
- Allergy Therapeutics (UK)
- DBV Technologies (France)
- HAL Allergy Group (Netherlands)
- Aimmune Therapeutics (U.S.)
Deciphering Mechanisms of Action and Cross-Reactivity
Asthma symptoms are influenced by various known risks and factors that can increase or decrease the likelihood of developing asthma. In addition, specific molecular mechanisms play a role in causing asthma. A perspective on and comprehension of asthma has evolved recently. Instead of focusing on individual factors, researchers now consider the exposome framework, which assumes imbalances in the body's microbial community, personal genetics, and unique environments when studying asthma and its treatments. The prevention and treatment of asthma symptoms can be addressed by understanding more about the genetics of diseases and the factors that lead to health issues.
Future Advancements: A Sneak Peek into Tomorrow's Therapies
Recently, there have been significant immunotherapy advancements, showing promising potential for future treatments. Novel and creative strategies are being developed to address safety issues and lower the chance of severe allergic reactions during immunotherapy. This includes the availability of various allergen preparations, including modified-recombinant allergens, recombinant allergens, and allergoids. Further studies are being conducted on virus-like particles, CpG motifs, and adjuvants like MPL and aluminium hydroxide, which have been shown to enhance immune responses and improve safety and effectiveness.
New methods in allergen immunotherapy involve applying extract patches on the skin or injecting them into the inguinal lymph nodes. By using recombinant technology or making chemical alterations to allergen molecules, they can become less reactive and potentially suppress certain immune responses or stimulate specific receptors. These advancements provide treatments that can modify the disease, prove cost-effective, and improve overall quality of life.
The future of Allergy Immunotherapy is moving towards precision medicine, which means tailoring treatments to individual patients. This approach emphasizes personalized and patient-specific strategies. While immunotherapy effectively prevents asthma development and new sensitizations, these positive outcomes are mainly observed in children. In contrast, adults require a different evaluation, with the primary focus being on alleviating allergic symptoms.
Buy Now Our Allergy Immunotherapy Industry Report @ https://www.vantagemarketresearch.com/buy-now/allergy-immunotherapy-market-2360/0
Regional Dynamics and Collaborative Endeavours
Allergy has become a significant public health crisis in Europe, affecting over 150 million people. According to estimates, 20% of children in Europe have asthma, 15% have allergic skin disorders, 8% have food allergies, and up to 30% have allergic rhinitis or conjunctivitis. In the upcoming decades, experts believe that more than half of Europeans may suffer from an allergy of some kind. The only available medical intervention that can significantly alter the course of the disease is allergen-specific immunotherapy. Numerous studies have demonstrated that Allergy Immunotherapy can change the course of the illness, lower long-term costs, and significantly enhance the quality of life for those who suffer from allergies.
The European government is acting to address allergies and promote individual and public health by:
Promoting awareness of allergen-specific immunotherapy
There is a need for awareness campaigns and educational programs to inform patients about the benefits of this treatment, especially for those who do not respond well to symptomatic drug treatments.
Updating healthcare policies to support allergen-specific immunotherapy By prioritizing this treatment in health planning and designing policies that provide financial support through national health insurance, the long-term effects of allergies can be reduced, resulting in cost savings and improved quality of life.
Prioritizing funding for research on allergen-specific immunotherapy
More research is needed to improve the diagnosis and treatment of allergies. European funding schemes should support research to unlock its full potential.
Monitoring the economic impact of allergies
It is essential to assess the costs and benefits of different allergy treatments, considering the large population affected. Treatments like allergen-specific immunotherapy, which addresses symptoms and long-term consequences, can be more cost-effective in the long run.
Streamlining allergy services
Access to allergy treatment varies across Europe. A broad spectrum of medical professionals should receive education and training to ensure that allergen-specific immunotherapy is accessible to those who require it.
Research Pioneers in Allergen Immunotherapy
In response to the escalating prevalence of allergies and the growing incidence of life-threatening emergencies, HAL Allergy is actively engaged in developing therapeutics. The company enhances the quality of life for allergic patients and meets professional customers' needs. HAL Allergy is dedicated to expanding its product portfolio by leveraging proprietary technology and specialized expertise. The main focus is creating a comprehensive, forward-looking pipeline addressing significant allergens. HAL Allergy is also committed to the continuous development of innovative therapeutics. The development program includes ongoing clinical trials for respiratory allergies (Ragweed, Mites) and food allergies (Peanut), strategically designed to address unmet medical needs and provide effective solutions for patients and healthcare professionals.
Clinical Trial Program
HAL Allergy is involved in a comprehensive clinical trial program focusing on globally relevant allergens. We have initiated a targeted development process involving clinical studies in human volunteers to address the pressing need for therapeutics in respiratory and food allergies. These trials aim to provide valuable insights into the safety and efficacy of our developed therapeutics.
The clinical trial process is divided into distinct phases, each with specific research goals:
Pre-clinical Trials: Conducted before entering human trials, this phase includes in-vivo testing.
Phase I Trials: These trials primarily assess the safety of a new medicine. Involving a small number of allergic patients, this initial phase focuses on potential side effects at different doses.
Phase II Trials: This phase is dedicated to testing the new medicine's safety and efficacy. Determining the optimal dosage is a key objective, and the study involves several hundred patients in a blinded setup, allowing for a careful and objective investigation.
Phase III Trials: Confirmatory; these trials investigate the safety and efficacy of the new medicine in larger populations, ranging from several hundred to several thousand patients, using the selected dose. This large-scale testing provides a comprehensive understanding of the effectiveness.
Conclusion
In conclusion, the evolving landscape of immunotherapy promises not only relief but a personalized approach that considers each patient's unique needs. The collaborative efforts of governments, research pioneers like HAL Allergy, and the scientific community pave the way for a future where allergies don't dictate our lives. A technological revolution in Allergy Immunotherapy is transforming the lives of millions affected by allergies worldwide.
Read Our Latest Press Release: Battery Recycling Market - In-depth Analysis
Contact us
Eric Kunz
6218 Georgia Avenue NW Ste 1 - 564
Washington DC 20011-5125
United States Tel: +1 202 380 9727
Email: [email protected]
Website: Vantage Market Research