Global Peanut Allergy Treatment Market Size to Reach $1278.3 Million at a CAGR of 11.6% by 2032
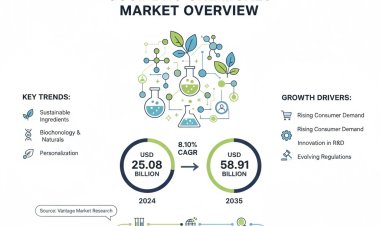
Vantage Market Research expects the Peanut Allergy Treatment Market to reach USD 1278.3 Million by 2032, exhibiting a growth rate (CAGR) of 11.6% during 2024-2032.

The Global Peanut Allergy Treatment Market size reached USD 478.2 Million in 2023. Vantage Market Research expects the market to reach USD 1278.3 Million by 2032, exhibiting a growth rate (CAGR) of 11.6% during 2024-2032.
Table of Contents
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Introduction
Peanut allergies, which impact millions of people globally, have caused a significant change in the Peanut Allergy Treatment Market. This condition, where the immune system reacts to even small amounts of peanuts, leads to unpredictable and severe allergic reactions, making it essential to develop advanced treatments. The market is experiencing a significant transformation as new research, clinical trials, and FDA approvals drive the development of innovative therapies. The treatment options range from traditional methods like antihistamines and epinephrine to new and ground-breaking approaches.
Request Sample Report of Peanut Allergy Treatment Market @ https://www.vantagemarketresearch.com/peanut-allergy-treatment-market-2428/request-sample
Top Companies in Global Peanut Allergy Treatment Market
- Alladapt Immunotherapeutics Inc. (U.S.)
- DBV Technologies (France)
- Sanofi (France)
- Vedanta Biosciences Inc. (U.S.)
- Aimmune Therapeutics Inc. (U.S.)
- Aravax Pty Ltd. (Australia)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Teva Pharmaceuticals Industries Ltd. (Israel)
The Prevalence and Impact
Peanut allergies are prevalent, affecting around 1 million children in the United States. According to the FDA, only about 1 in 5 children with this condition will outgrow it. According to the American Journal of Managed Care, 1.2% of Americans and nearly 2.5% of children in the country suffer from peanut allergy. It is the most common food allergy among kids and a significant cause of allergy-related deaths in children. Better healthcare insurance coverage has led to an even greater Peanut Allergy Treatment market expansion, underscoring the significance of creating workable solutions.
Biological Drugs: Catalyst for Transformation
The development of biological drugs is a hopeful opportunity to transform the Peanut Allergy Treatment Market. Trials conducted by Syneos Health on the Biological - Peanut SLIT tablet demonstrate the potential to lessen sensitivity to peanuts, with improved effectiveness observed with more extended treatment periods. These advancements indicate a new approach to addressing peanut allergies, potentially providing a safer and more efficient alternative.
Oral Immunotherapy: Advancements and Challenges
A new type of treatment called oral immunotherapy is becoming more popular in the medical field. In January 2020, the FDA approved a product for treating peanut allergies. While some people have raised concerns about the lack of standardized procedures and the high rate of adverse reactions, more and more people, especially young children, are choosing this form of treatment. Researchers are also exploring other immunotherapy methods, such as through the skin, under the tongue, under the skin, and within the lymph nodes. All of these options are contributing to the ever-changing landscape of allergy treatments.
Advancements in Clinical Trials
Clinical trials are significant for testing how safe and effective new treatments for peanut allergies are. Recent advancements from different trials, like the ones conducted by Regeneron Pharmaceuticals, Sanofi, and Alladapt Immunotherapeutics Inc., show a strong dedication to finding new and innovative solutions. The approval process from the FDA is being expedited, and positive results from studies are helping to move forward and make significant progress in the field.
Collaborations and Strategic Approaches
Collaboration across many groups is becoming more and more important in the treatment of peanut allergy in order to advance research, expedite the development process, and gain a deeper understanding of the intricate challenges involved. Healthcare providers, researchers, pharmaceutical companies, and regulatory bodies all recognize the importance of collective efforts to overcome the many challenges of peanut allergies. They are forming strategic partnerships to combine their expertise, share resources and knowledge, and use new technologies. By working together, these collaborations aim to speed up the development and availability of new treatments, ultimately benefiting patients more quickly.
Buy Now Our Peanut Allergy Treatment Industry Report @ https://www.vantagemarketresearch.com/buy-now/peanut-allergy-treatment-market-2428/0
Recent Approvals and Future Prospects
The recent advancements in the Peanut Allergy Treatment Market, such as the FDA's approval of Dupixent for pediatric patients with eosinophilic esophagitis and the fast-tracking of ADP101 by Alladapt Immunotherapeutics, Inc., highlight an important moment in addressing peanut allergies. These achievements demonstrate the industry's dedication to expanding treatment options and recognizing the importance of addressing food allergies, especially in children aged 4 to 17. With its focus on allergies to common food allergens, ADP101 has the potential to completely change the way of treating these allergies. Furthermore, ongoing studies like EPOPEX, which evaluates Viaskin Peanut in young children, show promising future possibilities and a commitment to assess long-term effectiveness and safety. The industry expects significant future breakthroughs and a better understanding of peanut allergy complexities through collaboration, strategic approaches, and supportive regulations that promote innovative and effective treatments.
Conclusion
The market for treating peanut allergies is undergoing a significant change driven by new ideas, partnerships, and recent government approvals. The industry is working hard to solve the problems posed by peanut allergies, and the combination of medical drugs, oral immunotherapy, and precise medicine technologies offers hope for better, personalized, and easier-to-access treatments. With ongoing research and development, the market is ready for even more progress, ultimately leading to better lives for people affected by peanut allergies.
Read Our Latest Press Release: Retort Pouch Market - In-depth Analysis
Contact us
Eric Kunz
6218 Georgia Avenue NW Ste 1 - 564
Washington DC 20011-5125
United States Tel: +1 202 380 9727
Email: [email protected]
Website: Vantage Market Research