Global Medical Device Outsourced Manufacturing Market Size to Reach $9.6 Billion at a CAGR of 14.5% by 2030
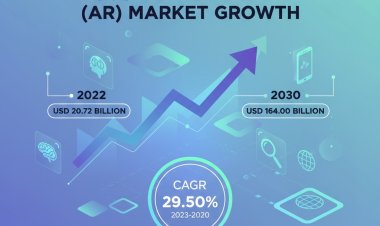
Vantage Market Research expects the Medical Device Outsourced Manufacturing Market to reach USD 76.8 Billion by 2030, exhibiting a growth rate (CAGR) of 14.5% during 2023-2030.

The Global Medical Device Outsourced Manufacturing Market size reached USD 29.8 Billion in 2022. Vantage Market Research expects the market to reach USD 76.8 Billion by 2030, exhibiting a growth rate (CAGR) of 14.5% during 2023-2030.
Table of Content [TOC]
|
Introduction |
|
What is Medical Device Outsourced Manufacturing? |
|
Why is Medical Device Outsourced Manufacturing Important? |
|
Drivers of Medical Device Outsourcing |
|
|
|
|
|
|
|
Benefits of Medical Device Outsourcing |
|
|
|
|
|
|
|
|
Conclusion |
In today's rapidly evolving healthcare landscape, Medical Device Outsourced Manufacturing plays a crucial role in enabling the development and delivery of innovative and high-quality medical technologies. As the medical device industry continues to grow, manufacturers are increasingly turning to outsourcing as a means to reduce costs, accelerate time to market, and enhance overall product quality.
Request Sample Report of Medical Device Outsourced Manufacturing Market @ https://www.vantagemarketresearch.com/medical-device-outsourced-manufacturing-market-2255/request-sample
Top Companies in Global Medical Device Outsourced Manufacturing Market
- SGS SA (Switzerland)
- Laboratory Corporation of America Holdings (U.S.)
- Euro fins Scientific (Luxembourg)
- Pace Analytical Services Inc. (U.S.)
- Intertek Group PLC (UK)
- WuXiAppTec (China)
- North American Science Associates LLC (U.S.)
- TUV SUD (Germany)
- Sterigenics U.S. LLC (GTCR LLC) (U.S.)
- Charles River Laboratories (U.S.)
- Medical Device Testing Services (U.S.)
- RJR Consulting Inc. (U.S.)
Healthcare providers and patients alike rely on medical devices for a wide range of applications, from diagnostic imaging and surgical instruments to implantable devices and wearable technologies. These devices must meet stringent regulatory standards to ensure safety, efficacy, and reliability. Medical Device Outsourced Manufacturing companies specialize in meeting these requirements, providing expertise in design, engineering, manufacturing, and quality control.
One of the primary drivers behind the outsourcing of medical device manufacturing is cost reduction. By partnering with a contract manufacturer, medical device companies can leverage economies of scale, access specialized equipment, and benefit from streamlined processes. This allows them to reduce production costs, which can then be passed on to healthcare providers and patients, ultimately improving affordability and accessibility of medical devices.
Moreover, outsourcing manufacturing also enables medical device companies to focus on their core competencies – research, development, and commercialization of innovative technologies. By entrusting the manufacturing processes to experts in the field, device companies can allocate their resources towards creating breakthrough products and advancing medical science. This collaboration between device companies and outsourced manufacturers fosters a synergistic relationship that drives innovation and enhances the quality of healthcare.
One of the major challenges in medical device manufacturing is regulatory compliance. Medical devices are subject to rigorous oversight by regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the European Union. Compliance with these regulations is essential to ensure patient safety and product efficacy.
Medical Device Outsourced Manufacturing companies have extensive experience navigating these regulatory landscapes. They possess the necessary expertise and infrastructure to adhere to the complex requirements imposed by regulatory bodies. By partnering with an outsourced manufacturer, medical device companies can ensure that their products meet all necessary regulatory standards, reducing the risk of costly delays in the approval process.
In addition to regulatory compliance, outsourced manufacturers also play a vital role in quality control. Medical devices must meet stringent quality standards to ensure accurate and reliable performance. Outsourced manufacturers employ robust quality control measures, including rigorous testing protocols and advanced quality management systems, to ensure that each device meets or exceeds its specifications.
An outsourced manufacturer's quality control processes often surpass the capabilities and capacities of in-house manufacturing. This is particularly important for complex devices that require specialized manufacturing techniques or cleanroom facilities. By partnering with an outsourced manufacturer, device companies can leverage their expertise, resources, and infrastructure to ensure the highest level of quality throughout the manufacturing process.
Outsourced manufacturing also offers benefits in terms of scalability and flexibility. The medical device industry is characterized by rapid technological advancement and changing market demands. For device companies, maintaining the flexibility to scale production volumes up or down quickly is essential to respond to market fluctuations and efficiently meet customer needs.
Buy Now Our Medical Device Outsourced Manufacturing Industry Report @ https://www.vantagemarketresearch.com/medical-device-outsourced-manufacturing-market-2255/request-sample
Outsourced manufacturers are well-positioned to accommodate these dynamic requirements. They have the capability to scale production volumes rapidly while maintaining consistent quality. This scalability allows device companies to respond to market demand without the need for significant investments in additional manufacturing capacity.
Moreover, by outsourcing manufacturing, device companies also gain access to a global network of suppliers and partners. This international reach not only provides cost advantages but also enables device companies to tap into a diverse pool of expertise, fostering innovation and knowledge exchange. Collaboration with outsourced manufacturers from different regions allows device companies to leverage cultural and technological diversity, driving continuous improvement and the development of cutting-edge technologies.
Conclusion
In conclusion, Medical Device Outsourced Manufacturing plays a crucial role in enabling innovation and quality in healthcare. By partnering with outsourced manufacturers, device companies can reduce costs, accelerate time to market, and enhance product quality. The expertise, infrastructure, and regulatory compliance capabilities of outsourced manufacturers provide device companies with the necessary support to create breakthrough medical technologies while ensuring patient safety and product efficacy. As the medical device industry continues its growth trajectory, the importance of Medical Device Outsourced Manufacturing is set to increase, driving further innovation and advancements in healthcare.
Read Our Latest Press Release: Specialty Silica Market - In-depth Analysis
Contact us
Eric Kunz
6218 Georgia Avenue NW Ste 1 - 564
Washington DC 20011-5125
United States Tel: +1 202 380 9727
Email: [email protected]
Website: Vantage Market Research