Global Cell and Gene Therapy Manufacturing Market Size to Reach $15.4 Billion at a CAGR of 18.2% by 2030
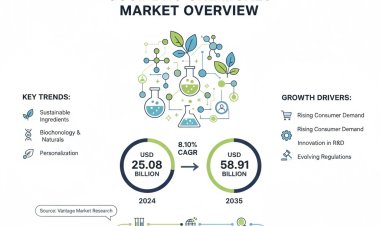
Vantage Market Research expects the Cell and Gene Therapy Manufacturing Market to reach USD 15.4 Billion by 2030, exhibiting a growth rate (CAGR) of 18.2% during 2023-2030.

The Global Cell and Gene Therapy Manufacturing Market size reached USD 4.8 Billion in 2022. Vantage Market Research expects the market to reach USD 15.4 Billion by 2030, exhibiting a growth rate (CAGR) of 18.2% during 2023-2030.
Table of Content [TOC]
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Evolution of Cell and Gene Therapy Manufacturing
Cell and Gene Therapy Manufacturing has emerged as a cutting-edge field, revolutionizing the treatment of diseases for which traditional therapies have been inadequate. This blog aims to provide insight into all aspects of this evolving field, covering topics ranging from the manufacturing process to regulatory challenges. By understanding the complexities involved in Cell and Gene Therapy Manufacturing, we can appreciate these therapies’ immense potential in improving patient outcomes.
Request Sample Report of Cell and Gene Therapy Manufacturing Market @ https://www.vantagemarketresearch.com/cell-and-gene-therapy-manufacturing-market-2227/request-sample
Top Companies in Global Cell and Gene Therapy Manufacturing Market
|
Company |
Revenue in 2022 |
Headquarters |
|
Lonza |
$6.2 billion |
Basel, Switzerland |
|
Bluebird Bio |
$3.6 million |
Cambridge, Massachusetts |
|
Catalent Inc. |
$5 billion |
Somerset, New Jersey |
|
F. Hoffmann-La Roche Ltd. |
$63 billion |
Basel, Switzerland |
|
Samsung Biologics |
KRW 2.44 trillion |
Incheon, South Korea |
|
Boehringer Ingelheim |
$24.1 billion euros |
Ingelheim, Germany |
|
Cellular Therapeutics |
$0.25 billion |
Beijing, China |
|
Hitachi Chemical Co. Ltd. |
10.88 trillion Yen |
Tokyo, Japan |
|
Takara Bio Inc. |
$304.1 million |
Shiga, Japan |
|
Miltenyi Biotec |
$845.3 Million |
Bergisch Gladbach, Germany |
|
Thermo Fisher Scientific |
$44.915 billion |
Waltham, Massachusetts |
|
Novartis International AG |
$51.828billion |
Basel, Switzerland |
|
Merck KGaA |
€22,232 million |
Darmstadt, Germany |
|
Wuxi Advanced Therapies |
RMB 39,355 million |
Wuxi, China |
|
Fujifilm Holdings Corp. |
$22.479 billion |
Tokyo, Japan |
|
Charles River Laboratories International Inc. |
$3.98 billion |
Wilmington, Massachusetts |
|
GE Healthcare Life Sciences |
$17.72 billion |
Chicago, Illinois |
|
CGT Catapult |
£74,000,000 |
London, England |
|
CoJourney Inc. |
N/A |
Shanghai, China |
|
ElevateBio LLC |
$100 million |
Cambridge, Massachusetts |
|
Pfizer Inc. |
$100.33 billion |
New York, New York |
|
Inc.te Corp. |
$16.30 billion |
Waltham, Massachusetts |
|
Allogene Therapeutics Inc. |
$0.24 million |
South San Francisco, California |
Understanding Cell and Gene Therapy
Cell therapy involves administering live cells to patients to restore or enhance their functional abilities. Conversely, gene therapy focuses on introducing or modifying genetic material to treat or prevent disease. This paragraph aims to briefly overview each therapy, highlighting their fundamental differences and illustrating their shared goal of targeted and personalized treatment options.
Manufacturing Processes in Cell and Gene Therapy
To achieve successful outcomes, precise manufacturing processes are crucial in cell and gene therapy. This section delves into the methodologies employed in manufacturing these therapies, encompassing everything from cell isolation and expansion to the engineering and delivery of therapeutic genes. Moreover, it explores the challenges, such as optimizing gene transfer efficiency, scaling up production, and ensuring quality control to meet regulatory standards.
Regulatory Considerations in Cell and Gene Therapy Manufacturing
The regulatory landscape plays a pivotal role in advancing Cell and Gene Therapy Manufacturing. This section sheds light on the guidelines set forth by regulatory bodies, such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). It addresses the specific requirements for manufacturing, including good manufacturing practices (GMP), quality control, and safety assessments. Furthermore, it discusses the regulatory hurdles manufacturers face and the need for consistent adherence to ensure patient safety and therapy efficacy.
Buy Now Our Cell and Gene Therapy Manufacturing Industry Report @ https://www.vantagemarketresearch.com/buy-now/cell-and-gene-therapy-manufacturing-market-2227/0
Challenges and Innovations in Scaling Up Manufacturing
As cellular and gene therapies progress toward commercialization, scaling up manufacturing processes presents technical and regulatory challenges. This paragraph explores the obstacles faced in transitioning from small-scale to large-scale production. It introduces innovations, such as automation and closed-system manufacturing platforms, to overcome these challenges and meet the increasing demand for these therapies. Additionally, it highlights the importance of developing standardized manufacturing protocols to ensure reproducibility and cost-effectiveness.
Future of Cell and Gene Therapy Manufacturing Market
Cell and Gene Therapy Manufacturing is continuously advancing, with exciting prospects for the future. This section discusses current research and development efforts focused on enhancing manufacturing strategies, including advanced bioreactors, novel gene delivery systems, and gene editing technologies like CRISPR-Cas9. Furthermore, it touches upon the potential for cell and gene therapies to extend beyond traditional applications, such as regenerative medicine and immuno-oncology.
Conclusion
In conclusion, Cell and Gene Therapy Manufacturing offers unprecedented possibilities for personalized and targeted treatment options. By understanding the intricacies of the manufacturing process, from cell isolation to regulatory requirements, we gain insight into how these revolutionary therapies are developed and brought to patients. While challenges remain, such as scaling up production and ensuring regulatory compliance, continuous innovation in manufacturing technologies promises to overcome these hurdles. As we look ahead, the future of Cell and Gene Therapy Manufacturing is teeming with potential, providing hope for the millions of patients worldwide in need of effective treatments and cures.
Frequently Asked Question (FAQ) – Cell and Gene Therapy Manufacturing Market
- What is cell and gene therapy manufacturing? Cell and gene therapy manufacturing refers to the process of producing therapeutic products that utilize cells or genes to treat various diseases. These therapies involve modifying or replacing a patient's cells or genes to restore normal function and combat diseases.
- How large is the cell and gene therapy manufacturing market? As of my last update in September 2021, the cell and gene therapy manufacturing market was rapidly growing, with substantial investments and advancements. However, for the most current market size and trends, I recommend checking the latest industry reports and market analysis.
- What are the key drivers of growth in this market? The cell and gene therapy manufacturing market is driven by factors like increasing prevalence of genetic disorders, rising investment in research and development, advancements in biotechnology, supportive regulatory environment, and a growing demand for personalized medicine.
- What challenges does the industry face? Challenges in the cell and gene therapy manufacturing market include complex manufacturing processes, high costs associated with development and production, regulatory complexities, scalability issues, and ensuring consistent quality control.
- Who are the major players in the cell and gene therapy manufacturing sector? Major companies involved in cell and gene therapy manufacturing include pharmaceutical giants, biotechnology firms, and contract manufacturing organizations (CMOs). Some notable players are Novartis AG, Kite Pharma (a Gilead company), bluebird bio, Lonza Group, and Catalent.
- How does regulatory approval impact cell and gene therapy manufacturing? Regulatory approval is crucial for cell and gene therapy products to ensure their safety, efficacy, and quality. Stringent regulatory processes, such as those by the FDA and EMA, are in place to assess these therapies before they can be marketed and administered to patients.
- What is the current focus in cell and gene therapy manufacturing research? Current research in this field focuses on improving manufacturing processes, enhancing scalability, reducing production costs, developing novel delivery methods, and addressing challenges related to immune responses and long-term effects.
- How accessible are cell and gene therapies to patients? Accessibility to cell and gene therapies can be limited due to factors like high costs, regulatory barriers, and specialized infrastructure requirements. Efforts are being made to address these challenges and improve patient access to these innovative treatments.
- Are there any geographic trends in the cell and gene therapy manufacturing market? The cell and gene therapy manufacturing market has a global presence, with active research and development hubs in regions like North America, Europe, and Asia. Different regions might have varying levels of investment, infrastructure, and regulatory frameworks supporting this industry.
Read Our Latest Press Release: CBD Nutraceuticals Market - In-depth Analysis
Contact us
Eric Kunz
6218 Georgia Avenue NW Ste 1 - 564
Washington DC 20011-5125
United States Tel: +1 202 380 9727
Email: [email protected]
Website: Vantage Market Research