Global Therapeutic Vaccines Market Size to Reach $140 Billion at a CAGR of 18.2% by 2032
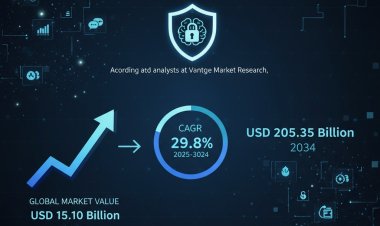
Vantage Market Research expects the Therapeutic Vaccines Market to reach USD 140 Billion by 2032, exhibiting a growth rate (CAGR) of 18.2% during 2024-2032.

The Global Therapeutic Vaccines Market size reached USD 31 Billion in 2023. Vantage Market Research expects the market to reach USD 140 Billion by 2032, exhibiting a growth rate (CAGR) of 18.2% during 2024-2032.
Table of Contents
|
|
|
|
|
|
|
|
|
|
|
Request Sample Report of Therapeutic Vaccines Market @ https://www.vantagemarketresearch.com/therapeutic-vaccines-market-2384/request-sample
Top Companies in Global Therapeutic Vaccines Market
- Agenus Inc. (U.S.)
- Argos Therapeutics Inc. (U.S.)
- Bavarian Nordic A/S (Denmark)
- Cel-Sci Corp. (U.S.)
- CSL Ltd. (Australia)
- Emergent Biosolutions Inc. (U.S.)
- GSK PLC (UK)
- Merck & Co. Inc. (U.S.)
- Pfizer Inc. (U.S.)
The Burden of Chronic Non-Communicable Diseases
Globally, infectious illnesses remain the leading motive of mortality. Conventional vaccinations in opposition to ailments, including measles, polio, diphtheria, tetanus, and smallpox, have succeeded in stopping and lessening their effects. Nonetheless, some of the different infectious diseases, along with influenza, HBV, HIV, HCV, and HPV, still result in excessive death. These days, persistent non-communicable diseases—which account for 71% of all fatalities and place a heavy financial burden on society worldwide—are the primary reasons for illness and mortality. Conditions like coronary heart disorder, cancers, respiration ailments, diabetes, excessive blood strain, Alzheimer's ailment, excessive cholesterol, bronchial asthma, and chronic obstructive pulmonary disorder contribute to over 80% of all deaths. Therefore, there may be a need for healing vaccines that can enhance the body's immune reaction and successfully treat infected diseases. These vaccines activate specific immune responses that target and eliminate infected cells.
About Therapeutic Vaccines
Therapeutic vaccines have emerged as a promising way to address both infectious and continual non-communicable illnesses. These vaccines are designed for prevention and stimulate specific immune responses, breaking the body's immune tolerance and targeting established infections. Three primary forms of healing vaccines are being explored in the preclinical and clinical phases: molecular-based, vector-based, and cell-based.
- Molecular-Based Vaccines: Molecular-based vaccines encompass peptide/protein, DNA, and mRNA. These vaccines utilize neoantigens, purified peptides/proteins, or DNA/mRNA-encoded proteins in combination with adjuvants to elicit immune responses.
- Vector-Based Vaccines: Vector-based vaccines leverage naturally or genetically engineered bacteria, viruses, and yeast as carriers to express antigen transgenes. This approach provides an effective means to trigger immune responses.
- Cell-Based Vaccines: Cell-based vaccines include dendritic and genetically modified cell vaccines, utilizing dendritic or genetically modified cells to express and deliver antigens.
Therapeutic vaccines aim to maximize the induction of epitope-specific T cells or B cells, capable of targeting and eliminating infected cells or producing specific antibodies to neutralize viruses.
The Positive Clinical Role of Therapeutic Vaccines
Numerous researches, as highlighted by means of the National Institute of Health, attest to the positive scientific impact of healing vaccines in treating tumours and infectious diseases. This progress is evident in the increasing number of therapeutic vaccines transitioning from basic laboratory research to clinical trials. Compared to standard drugs, these vaccines offer benefits including excessive specificity, minimum facet results, long-lasting results, and a reduced likelihood of drug resistance.
Recent Advancements
Personalized Therapy and Immunomodulation: Recent advancements in therapeutic vaccines are steering the field toward personalized therapy. The dynamic immune response induced by therapeutic vaccines allows for ongoing adaptation and expansion, contributing to a more patient-relevant immune response. Clinical studies have provided information about the therapeutic action of vaccines, highlighting their potential as the gold standard for individualized treatment.
Monoclonal Antibodies, Modern Vaccines, and Gene Therapy: The intersection of biotechnology, biology, and engineering has given upward push to groundbreaking trends. Monoclonal antibodies, contemporary vaccines, and gene therapy have become key player in the biotechnological landscape.
Genetic engineering: Using biotechnology, genetic engineering, additionally called genetic amendment, entails at once modifying an organism's genome. This includes inserting new DNA, removing or "knocking out" genes, and using gene targeting techniques for precise modifications. Recombinant nucleic acid strategies are utilized in genetic engineering to change an organism's genetic composition and convey novel combos of heritable genetic material.
Strategies to Enhance Therapeutic Vaccines
Diverse therapeutic vaccine strategies are currently under development or evaluation in clinical trials. These strategies can be categorized into cell-based, subunit, and genetic vaccines. Overcoming tumour-related immunosuppression is a primary goal, which involves optimizing vaccines in terms of antigen choice, immunological adjuvants, delivery formulations, efficacy, safety, and toxicity considerations. Moreover, preclinical research underscores the ability of combining vaccines with different immunotherapies, such as immune checkpoint blockade, to enhance therapeutic outcomes.
Buy Now Our Therapeutic Vaccines Industry Report @ https://www.vantagemarketresearch.com/buy-now/therapeutic-vaccines-market-2384/0
Competitive Landscape
As of August 2023, several notable developments have shaped the competitive landscape of the therapeutic vaccines market:
- Project NextGen: The U.S. Department of Health and Human Services allocated over $1.4 billion for Project NextGen, a $5 billion initiative to advance innovative vaccines and therapeutics, particularly for COVID-19. This initiative focuses on developing tools and technologies for years to protect against the virus.
- Dupixent Approval: Dupixent is certified with the aid of the U.S. FDA ( Food and Drug Administration) for kids with slight-to-extreme atopic dermatitis who're between the age of 6 months and 5 years. This marks a significant step in providing effective treatment options for a younger age group.
- ABRYSVO™ Authorization: Pfizer Inc. received marketing authorization from the European Commission for ABRYSVO™, a bivalent respiratory syncytial virus (RSV) prefusion F (RSVpreF) vaccine. This vaccine protects infants and older adults against RSV through maternal immunization.
- LYNPARZA Approval: The FDA has approved LYNPARZA when taken with abiraterone and prednisone or prednisolone for the treatment of adult patients with metastatic castration-resistant prostate cancer with a BRCA mutation, according to a statement released by AstraZeneca and Merck. This approval is based on positive outcomes from the Phase 3 PROpel trial.
Conclusion
The evolving landscape of therapeutic vaccines reflects a paradigm shift in addressing infectious and chronic non-communicable diseases. Novel vaccination approaches have been made possible by developments in immunology, genetic engineering, and molecular biology. As the field progresses, integrating personalized therapy, immunomodulation, and combination therapies holds the promise of more effective and targeted treatments. With ongoing research and strategic investments, the therapeutic vaccines market is poised to play a pivotal role in shaping the future of healthcare.
Read Our Latest Press Release: Anticoccidial Drugs Market - In-depth Analysis
Contact us
Eric Kunz
6218 Georgia Avenue NW Ste 1 - 564
Washington DC 20011-5125
United States Tel: +1 202 380 9727
Email: [email protected]
Website: Vantage Market Research