Cardiogenic Shock Treatment Market 2024-2034 | Size, Share, Trends
Explore the growing cardiogenic shock treatment market. Learn about key drivers, challenges, regional insights, and leading companies in this sector.
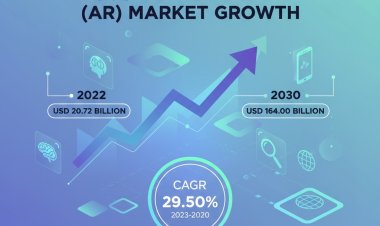
The global market for cardiogenic shock treatment reached USD 1.08 billion in 2024 and is projected to grow at a compound annual growth rate (CAGR) of 6.9% between 2025 and 2034, reaching USD 2.10 billion by 2034. This market segment within the healthcare industry is focused on the effective management of cardiogenic shock, a serious condition.
Vantage Market Research offers a concise overview of the current market size and provides projections for its anticipated growth over the next decade. The report identifies key drivers of market growth, including the rising incidence of heart disease, particularly in South and East Asia, and the increasing adoption of mechanical support devices like Impella and ECMO. Furthermore, the report examines the challenges associated with the high cost of treatment, which can limit access to these advanced therapies. The report provides further insight into the key market segments, with a particular focus on the dominant role of hospitals in treatment and the increasing prevalence of medical devices, such as Impella and ECMO, in managing cardiogenic shock. In conclusion, the report outlines the key market players and highlights recent developments, including the establishment of the Cardiogenic Shock Registry by the American Heart Association.
Request Sample Report of Cardiogenic Shock Treatment Market @ https://www.vantagemarketresearch.com/cardiogenic-shock-treatment-market-3317/request-sample
Market demand analysis and forecast 2018-22 for cardiopulmonary treatment of cardiogenic shock to 2033.
From 2018 to 2022, the cardiogenic shock treatment market demonstrated a 3.5% annual growth rate. This growth is attributed to the increasing use of mechanical support devices like Impella and ECMO, which enhance blood circulation in patients experiencing cardiogenic shock. Moreover, the emergence of new treatment options, including stem cell and gene therapy, is poised to further drive market expansion. The market is projected to continue its growth trajectory from 2023 to 2033, with an anticipated CAGR of 7% during this period. This growth is attributed to a number of factors, including demographic trends, technological advancements, and economic conditions. The increasing incidence of cardiogenic shock is expected to drive demand for effective treatments.

Track market trends LIVE & outsmart rivals with our Premium Data Intel Tool: Vantage Point
The following factors are key drivers of the cardiopulmonary bypass (CPB) market:
Rising Incidence of Heart Disease
The rising prevalence of heart disease is a key driver of the cardiogenic shock treatment market's expansion. Heart disease is a significant global health concern, with a number of contributing factors including lifestyle choices, genetic predisposition, and underlying medical conditions such as hypertension and diabetes. Cardiogenic shock, frequently precipitated by a myocardial infarction, represents a grave complication of cardiovascular disease. As the prevalence of heart disease rises, so too does the incidence of cardiogenic shock. The American Heart Association estimates that approximately 805,000 Americans experience heart attacks annually, with 7-8% of these cases developing into cardiogenic shock. It should be noted that other cardiovascular conditions, such as heart failure, arrhythmias, and valve disorders, can also contribute to cardiogenic shock. As the population ages and these conditions become more prevalent, we can anticipate an increase in the incidence of cardiogenic shock. This increasing incidence is driving the demand for effective cardiogenic shock treatments, which is in turn propelling market growth. The advancement of mechanical support devices, such as the Impella and ECMO, has markedly enhanced the outlook for patients with cardiogenic shock. The emergence of novel treatment avenues, including stem cell and gene therapy, is poised to further propel market expansion.
The cardiogenic shock treatment market faces challenges.
High Treatment Costs
The financial implications of treating cardiogenic shock, particularly when utilising mechanical support devices such as Impella and ECMO, can be significant. The high cost of these treatments may restrict access for some patients and potentially hinder market growth. Furthermore, the lengthy and costly process of developing and obtaining approval for new cardiogenic shock treatments can restrict the availability of novel options and contribute to market stagnation.
Regional Insights: The South and East Asian markets
The market for cardiogenic shock treatment is experiencing growth in South and East Asia, driven by the rising prevalence of cardiovascular diseases. This rise is attributed to factors such as changing lifestyles, unhealthy diets, and high pollution levels in countries like India, China, and Japan. Consequently, the number of patients with cardiogenic shock is increasing, leading to a greater demand for effective treatment options.
The aging population and improving healthcare infrastructure present a significant opportunity for growth in the healthcare sector.
The growing aging population in South and East Asia represents an additional factor contributing to market growth. As people age, their risk of developing cardiovascular diseases and other chronic conditions increases, resulting in a higher demand for healthcare services, including cardiogenic shock treatment. Furthermore, enhancements in healthcare infrastructure across numerous countries in the region, including investments in medical facilities, equipment, and access to sophisticated treatments, are expanding the availability of cardiogenic shock treatment options. This is driving the number of hospitals and medical centers offering these treatments up, which is contributing to market expansion.
Regional Insights: The North American market
Prioritize technological advancements.
Analysts project that North America will maintain a dominant market position, accounting for approximately 40% of the total market share during the forecast period. The region has one of the highest global incidence rates of cardiovascular disease, with heart attacks being a leading cause of death. This high incidence has led to a growing demand for effective treatment options for cardiogenic shock. Notably, North America has been at the forefront of developing and utilizing mechanical support devices such as Impella and ECMO, which have become increasingly common in treating cardiogenic shock. These devices have considerably improved the prognosis for patients with cardiogenic shock and are driving market growth.
The following section provides insights on the categories in question. End-User Segment
Hospitals will represent a significant share of the market.
Hospitals are positioned to play a pivotal role in the cardiogenic shock treatment market, given their status as the primary care providers for patients with this condition. Hospitals are equipped with the necessary medical equipment and trained personnel to provide critical care and interventions, which is why they are well-positioned to treat cardiogenic shock. A significant number of hospitals have established specialized cardiac care units or departments with the objective of providing optimal treatment for cardiovascular diseases, including cardiogenic shock. Furthermore, hospitals are the primary sites for implanting medical devices like Impella, ECMO, and IABPs, which require specialized training and expertise. While cardiac centers and ambulatory surgical centers also play a role in treating cardiogenic shock, they typically offer more specialized services and may not provide the same level of critical care as hospitals. Therefore, we anticipate that hospitals will continue to dominate the cardiogenic shock treatment market in the coming years.
The following section will provide insights on the market by category.
The market growth is driven by the use of devices in treatment.
The use of medical devices such as the Impella, ECMO, and intra-aortic balloon pumps (IABPs) is on the rise in the treatment of cardiogenic shock. These devices provide mechanical support to the heart, restore blood flow, and deliver oxygen to the body's organs, resulting in significantly improved survival rates and outcomes for patients. Furthermore, additional medical devices and technologies, including extracorporeal carbon dioxide removal (ECCO2R) devices, microaxial flow pumps, and novel ventricular assist devices (VADs), are currently in development and undergoing testing for the treatment of cardiogenic shock. It is anticipated that these developments will further improve patient outcomes and stimulate growth in the medical devices sector of the market. While medications and surgery remain important treatment options, they are typically used in conjunction with medical devices rather than as standalone treatments. It is therefore anticipated that the medical devices segment will become the dominant force in the cardiogenic shock treatment market in the coming years.
Market Competition
Key players in the cardiogenic shock treatment market include companies like Getinge AB, Par Pharmaceutical, Abbott, Viatris Inc., F. Hoffman-La Roche Ltd, Bayer AG, Terumo Corporation, Medtronic, AbioMed, Astrazeneca, healthcare providers, and technology companies.
Company List:
- Abbott
- Abiomed
- Medtronic
- Boston Scientific Corporation
- Getinge AB
- Terumo Corporation
- Chiesi Farmaceutici S.p.A.
- Xenios AG (part of Fresenius Medical Care)
- ZOLL Medical Corporation
- Windtree Therapeutics
- Eli Lilly and Company
- Ferrer Internacional S.A.
- Novo Nordisk A/S
- Bayer AG
- Baxter International Inc.
Recent Developments:
In October 2022, the American Heart Association established the Cardiogenic Shock Registry, powered by Get With The Guidelines®, to enhance the understanding of cardiogenic shock symptoms, treatment patterns, and outcomes. This registry, building on over two decades of experience with the Get With The Guidelines® platform, will provide valuable information to researchers, clinicians, and regulators on the best treatment approaches for cardiogenic shock. The registry's steering committee comprises experts from leading academic surgeons and cardiologists, along with representatives from the USA Food & Drug Administration and the USA Centers for Medicare & Medicaid Services. These experts provide guidance on data establishment and management within the registry.
Cardiogenic Shock Treatment Market Segmentation:
By Product Type
- Mechanical Circulatory Support Devices
- Extracorporeal Membrane Oxygenation (ECMO)
- Intra-Aortic Balloon Pumps
- Pharmacological Treatment
- Vasopressors
- Inotropes
- Monitoring Devices
- Others
- Catheters
- Stents
By Indication
- Acute Myocardial Infarction-Induced Cardiogenic Shock
- Heart Failure-Induced Cardiogenic Shock
- Post-Cardiotomy Shock
- Others
- Sepsis-Induced Cardiogenic Shock
By Treatment Type
- Medication-Based Therapy
- Device-Based Therapy
- Combination Therapy
- Supportive Care and Monitoring
By End User
- Hospitals
- Cardiac Care Centers
- Ambulatory Surgical Centers
- Specialty Clinics
Frequently Asked Questions:
Q: What is the global market size for cardiogenic shock treatments in 2024 and the projected size for 2034.
- A: The global cardiogenic shock treatment market size was calculated at USD 1.08 billion in 2024 and is projected to reach USD 2.10 billion in 2034.
Q: Which regional market accounted for the largest revenue share in 2023, and what is the anticipated trend over the forecast period?
- A: Our analysis indicates that North America will account for the largest revenue share in the global market over the forecast period.
Q: Which companies are included in the global cardiogenic shock treatment market report?
- A: The following companies are major players in the market: Abbott, Abiomed, Medtronic, Boston Scientific Corporation, Getinge AB, Terumo Corporation, Chiesi Farmaceutici S.p.A., Xenios AG (Fresenius Medical Care), ZOLL Medical Corporation, Windtree Therapeutics, Eli Lilly and Company, Ferrer Internacional S.A., Novo Nordisk A/S, and Bayer AG, Baxter International Inc.
Q: What is the projected revenue compound annual growth rate (CAGR) of the global Cardiogenic Shock Treatment market over the forecast period?
- A: The global cardiogenic shock treatment market is projected to register a compound annual growth rate (CAGR) of 6.9% between 2025 and 2034.
Q: What are the primary drivers of revenue growth in the cardiopulmonary bypass market?
- A: The market revenue growth is driven by several key factors, including the rising incidence of acute heart conditions, advancements in medical technology, the increasing use of innovative mechanical circulatory support devices such as Impella pumps and Extracorporeal Membrane Oxygenation (ECMO), a rising focus on minimally invasive procedures, and a growing trend of intervention strategies, including advanced drug therapies that improve cardiac function.
☎ Contact us:
Vantage Market Research
224 W 35th St Ste 500 New York,
NY 10001 United States
United States Tel: +1 (212) 951-1369
✉ Email: [email protected]
☞ Website: https://www.vantagemarketresearch.com